Description
Isotopes are variants of a particular chemical element that have the same number of protons (and thus belong to the same element) but differ in the number of neutrons in their nuclei. This difference in neutron count results in isotopes of an element having different atomic masses. For example, carbon has three naturally occurring isotopes: carbon-12, carbon-13, and carbon-14. All carbon atoms have six protons, but carbon-12 has six neutrons, carbon-13 has seven neutrons, and carbon-14 has eight neutrons.
Isotopes exhibit nearly identical chemical behavior because chemical properties are primarily determined by the electron configuration, which is the same for all isotopes of an element. However, their physical properties can differ significantly. For instance, heavier isotopes tend to have slightly higher boiling and melting points.
Some isotopes are stable, while others are unstable, or radioactive. Radioactive isotopes, like carbon-14 or uranium-235, undergo radioactive decay, leading to the emission of radiation as they transform into more stable forms. This property makes them useful in various applications, such as radiometric dating in archaeology and geology, medical imaging and treatments in healthcare, and as tracers in biological and environmental studies.
The study of isotopes has wide-ranging implications in science, from understanding elemental and cosmic origins to practical applications in medicine, energy, and environmental science. The diversity of isotopes enriches our understanding of the atomic world, offering unique insights into the structure, history, and processes of matter.
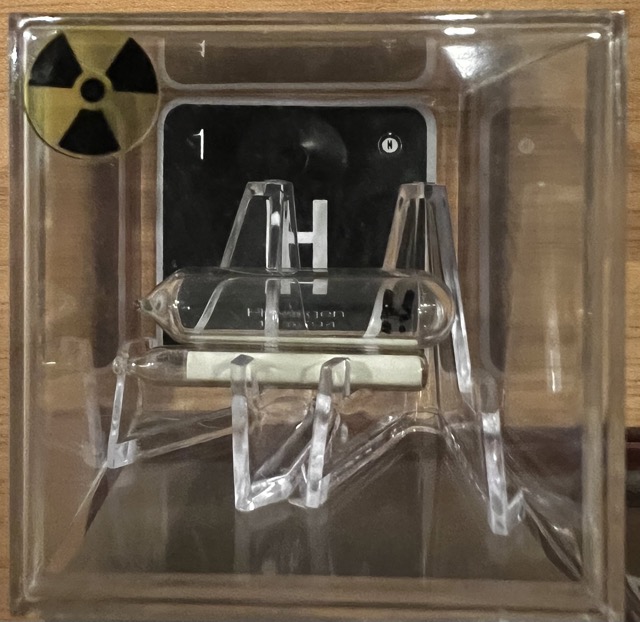
Tritium
Tritium, often symbolized as 3H or T, is a radioactive isotope of hydrogen. Unlike the most common[…]
 using WordPress and
using WordPress and