Since Dmitri Mendeleev unveiled his periodic table of elements in 1869, it has become a central pillar in the study of chemistry. However, the beauty of science lies in its constant evolution and willingness to embrace new ideas. Over the years, several alternate tables have been proposed, each offering a unique perspective on the organization and relationship of the elements. In this blog post, we will explore some of these alternate tables and the motivations behind their creation.
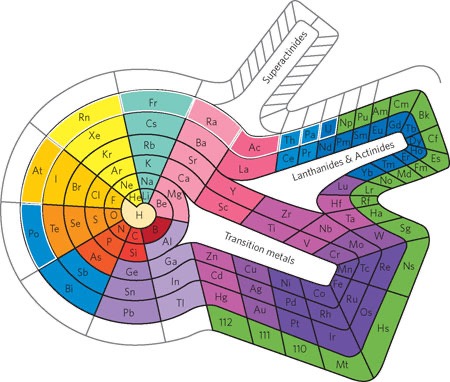
The Spiral or Helical Periodic Table
One of the most visually striking alternatives is the spiral or helical periodic table. Proposed by Theodor Benfey in the 1960s, this table arranges the elements in a spiral on a two-dimensional plane or in three dimensions. It starts with hydrogen at the center and spirals outwards, maintaining the group and period relationships. This design visually emphasizes the periodicity of the elements and can be particularly helpful in highlighting trends and properties across different periods and groups.
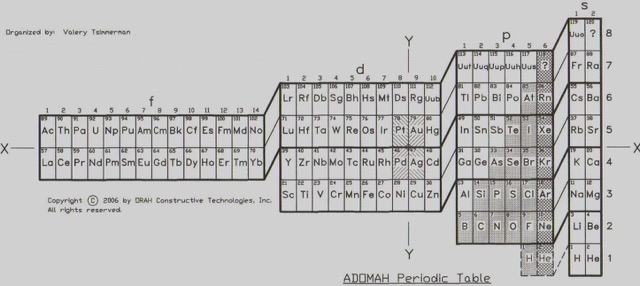
The ADOMAH Periodic Table
Developed by Valery Tsimmerman, the ADOMAH is another intriguing variation. It is based on the electron configuration of atoms, arranging elements in a way that aligns with their electron shells. This format is especially insightful for understanding the quantum mechanical underpinnings of the elements’ behavior and properties.
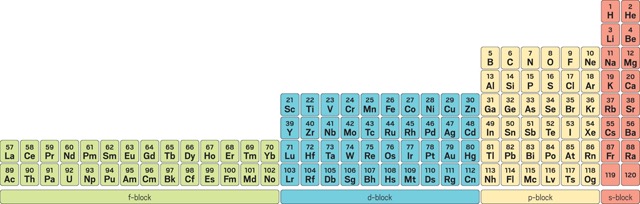
The Left-Step Periodic Table
The Left-Step Periodic Table, proposed by Charles Janet in the 1920s, is a significant departure from the traditional layout. It places the s-block elements on the right and shifts the other blocks accordingly. This arrangement can provide a clearer understanding of electron configurations and offers a more logical grouping of elements with similar properties.
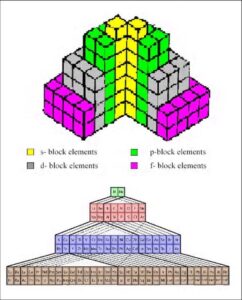
The Three-Dimensional Periodic Tables
Moving beyond two dimensions, some scientists have proposed three-dimensional periodic tables. These models aim to represent the elements in a form that more accurately reflects their atomic and molecular structures. While visually complex, these 3D tables can offer deeper insights into the relationships between elements, particularly in terms of their chemical bonding and reactivity.

The Mayan Periodic Table
A more recent and culturally inspired version is the Mayan Periodic Table, created by Mexican chemist Fernando Dufour. It arranges the elements in a circular format inspired by the Mayan calendar, aiming to create a more intuitive understanding of the periodic relationships and electron configurations.
Conclusion
While Mendeleev’s design remains the standard in education and research, these alternate periodic tables highlight the dynamic and multifaceted nature of chemistry. Each alternative brings a unique perspective, whether it’s emphasizing electron configurations, highlighting periodic trends, or offering new ways to visualize the complex relationships between elements. The exploration of these alternate tables is not just an academic exercise but a testament to the ongoing quest for knowledge and understanding in the ever-evolving field of chemistry.
 using WordPress and
using WordPress and
No responses yet